Which of These Best Describes Temperature of a Substance
Now as heat is added evenly at a steady rate to the substances of same masses therefore heat added rate time. The triple point best describes the only temperature and pressure at which all three states of a substance coexist in equilibrium.

For Any Substance Why Does The Temperature Remain Constant During The Change Of State
The water evaporated from the fishing gear and sand was attracted to it.

. ОАЕ Two substances at different temperatures are brought together and allowed to reach thermal equilibrium. If we see graph we find that in solid phases of substances in graph P for a smaller change in time temperature change is larger than graph. It is known that 158 x 10 5 J of heat energy are absorbed by the pan to reach the desired temperature and the specific heat of iron is 0450 Jg o C.
For optimal frying the pan must be heated to about 178 o C from a room temperature of 220 o C. Following are descriptions of the two types of properties. A a gas under constant volume B a liquid C a solid D a substance undergoing phase change.
The temperature at which a substance boils. Later he noticed that small white crystals had formed on the rod reel and fishing line. Expert answered alfred123 Points.
A substances specific heat tells you how much heat must be added to removed from a 1-g sample of this substance in order to get its temperature to change by 1C. Substance undergoing a change of state. In the sun to dry.
A 319 B 395 C 592 D 668 16A liquids freezing point is 38ºC and its boiling point is 357ºC. Physics questions and answers. Substance C is very hard does not conduct electricity and has a melting point of 3440 C.
The dry ice converts directly to gas leaving the solution. Which statement best describes the final temperature of the two substances. Which of these best describes the states of the substance represented by state z.
Temperature is the measure of the average amount of kinetic energy in a substance. At Time 1 the atoms are close together. 125 g of dry ice solid CO2 is dropped into a beaker containing 500 g of 66C water.
The mass and volume of these two chunks are different extensive properties but the color is the same. The average kinetic energy in a substance is called Select one. Above the critical temperature a substance.
The specific heat of a substance is the amount of heat required to raise the temperature of unit mass of substance through 1 o. Unit 7 Practice Test copy DRAFT. Some examples of physical properties are.
A substance made up of two or more elements that have been chemically combined is called. Which statement accurately describes a type of potential energy found in a container full of a chemical substance in liquid form. Which of the following best describes a substance in which the temperature remains constant while at the same time it is experiencing an inward heat flow.
The temperature of the substance increases by 201 degrees Celsius when 126 J of heat is added to the substance. Help me check my work. When the dry ice is gone the final.
Which of these best describes vaporization. Particles are not free to move about. The diagram shows the arrangement of atoms of the same substance at two different times.
All of the above none of the above. These properties enable a substance to change into a brand-new substance and they describe how a substance reacts with other substances. Temperature is the measure of the heat of an object.
Which of these choices best describes the thermal energy of a substance. Which of these choices best describes the thermal energy of a substance. D is boiling point the temperature at which a substance changes its state.
Which statement BEST describes the change occurring from Time 1 to Time 2. Which of the following best describes a substance whose temperature remains constant while at the same time it is experiencing an inward heat flow. The dry ice converts directly to gas leaving the solution.
The mass of substance B is four times the mass of substance A. Substance A has a specific heat capacity that is two times that of substance B. What is the number of Kelvin between the boiling point and the freezing point of the liquid.
A cast iron skillet is used to fry bacon. The physical state of a substance depends on the arrangement of atoms or molecules within it. What determines the average kinetic energy of the molecules of any gas.
At Time 2 the atoms are moving apart. The rotation of the particles is one place where potential energy is stored. Physical properties are properties that can be measured or observed without changing the chemical nature of the substance.
Vibrate about fixed positions. Temperature of the water is 29C. 125 g of dry ice solid CO2 is dropped into a beaker containing 500 g of 66C.
Substance B is brittle does not conduct electricity as a solid but does when molten and has a melting point of 2072 C. Statement correctly describes the energy changes between A and B. Which of these is the best explanation of what occurred.
A it is a solid because strong attractive forces prevent particles from moving. Which of these choices best describes the temperature of a. The table compares the characteristics of a substance in three different states of matter.
Which of the following best describes temperature. A the sum of the kinetic energy of all of the particles of a substance b the average kinetic energy of the particles that make up a substance c the total. The temperature at which a substance melts.
Temperature is the measure of the total amount of energy in a substance. C 283 D 293 17The temperature of a sample of a substance changes. Substance A is malleable ductile conducts electricity well and has a melting point of 1135 C.
Chemistry questions and answers. When the dry ice is gone the final temperature of the water is 29C. When energy in the form of heat is absorbed a substances specific heat tells you exactly how much heat is needed in order to increase the temperature of 1 g of this substance by.
What must the mass of the skillet be.

Specific Heat Definition Facts Britannica

Synthesis Physical Vs Chemical Properties Changes Physical Vs Chemical Properties Physics Teaching Chemistry
Why Does The Temperature Of A Substance Remain Constant At Its Boiling And Melting Points Quora
Solved Question 25 1 Point When Solid Ammonium Chloride Chegg Com

Physical Chemical Properties Changes

Exothermic Vs Endothermic Worksheet Change 1 When Calcium

The Given Graph Shows The Heating Curve For A Pure Substance The Temperature Rises With Time As The Substance Is Heated A What Is The Physical State Of The Substance At
Why Does The Temperature Of A Substance Remain Constant At Its Boiling And Melting Points Quora

Grade 7 Heat And Temperature Unit Test
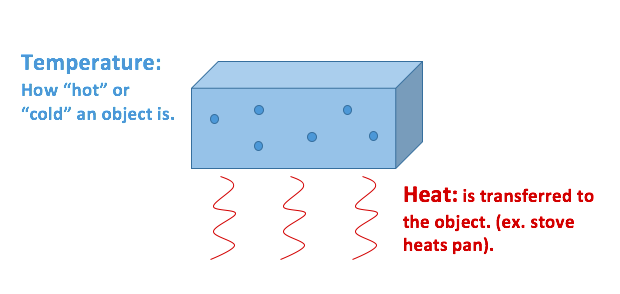
Heat Vs Temperature Energy Education

Distance Learning Science Ngss Assessment Tasks Ms Ps1 4 Adding Removing Heat Learning Science Ngss Physical Science

Temperature And Rate Of A Chemical Reaction Chapter 6 Chemical Change Middle School Chemistry

Temperature Dependence Of The Rate Of A Reaction Arrhenius Equation

For The First Three Questions Use The Key Below

Physics U10 Heat Flashcards Quizlet

Chapter 2a Pure Substances Phase Change Properties Updated 9 20 09
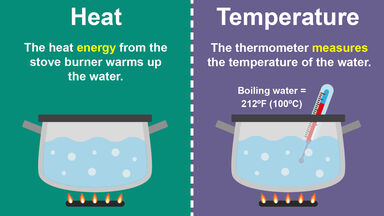
Difference Between Heat And Temperature In Simple Terms
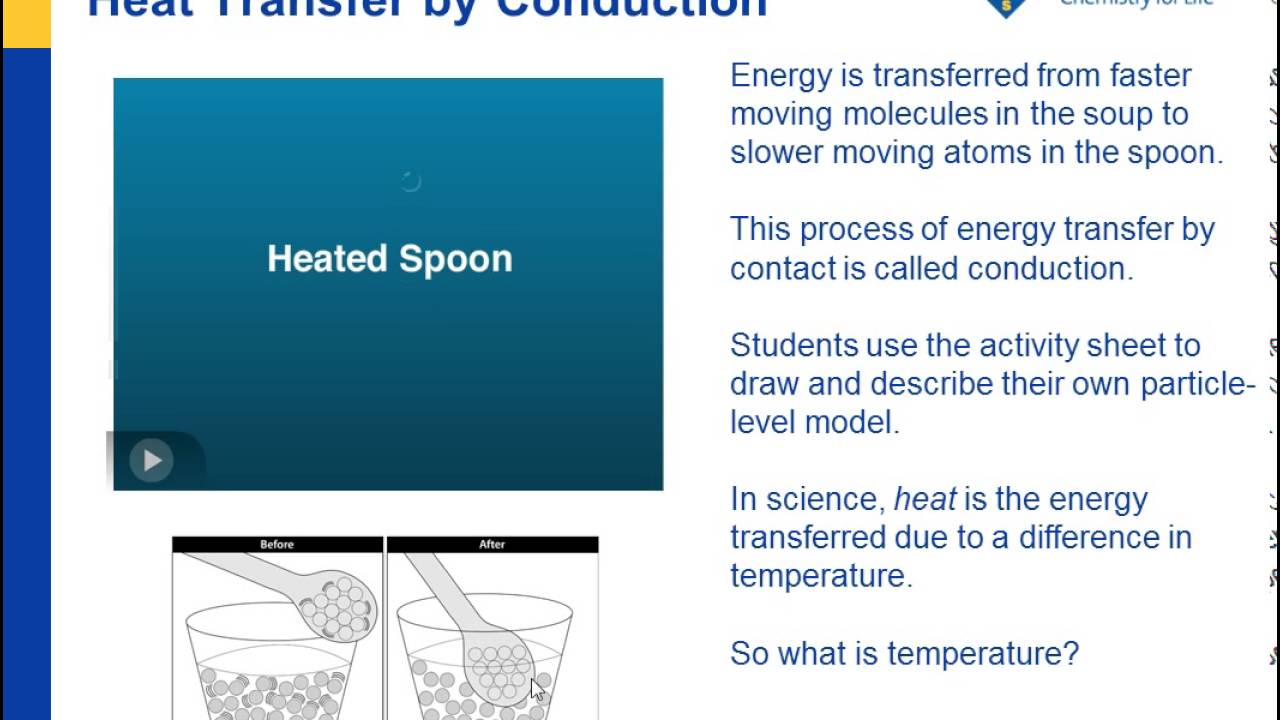
Heat Temperature And Conduction Chapter 2 States Of Matter Middle School Chemistry

Comments
Post a Comment